If pressure and volume are held constant, what is the effect of increasing the number of moles of an ideal gas in a closed container?
A. increasing the number of moles of gas has no effect
B. the temperature in the container increases
C. the temperature in the container decreases
D. the effect of increasing the number of moles of ideal gas cannot be determined
Click for Explanation
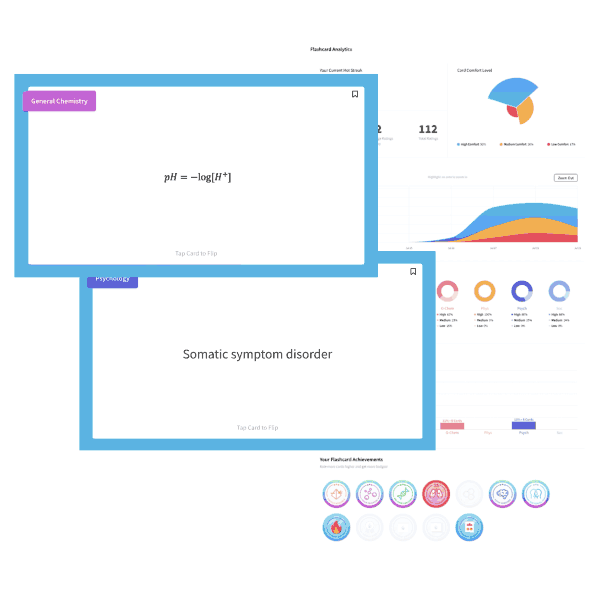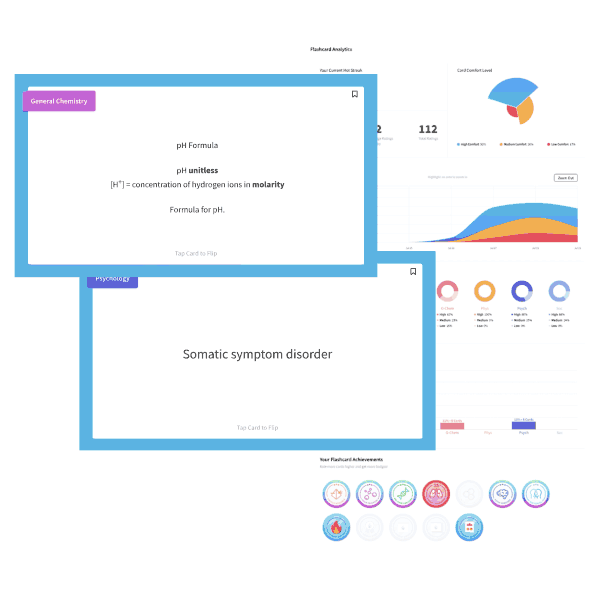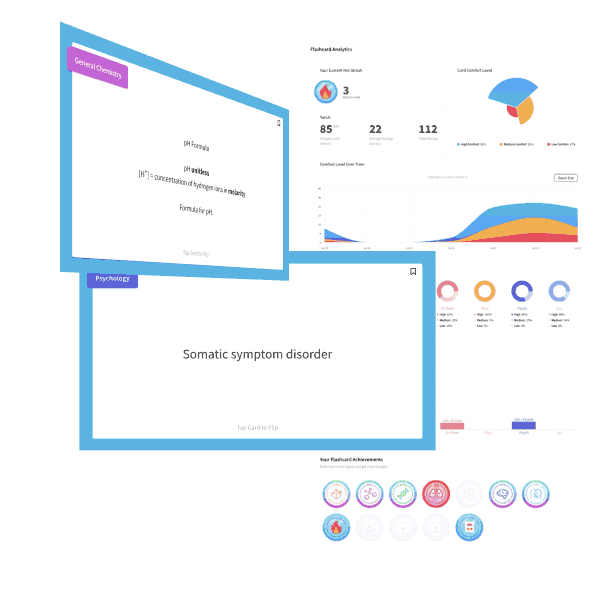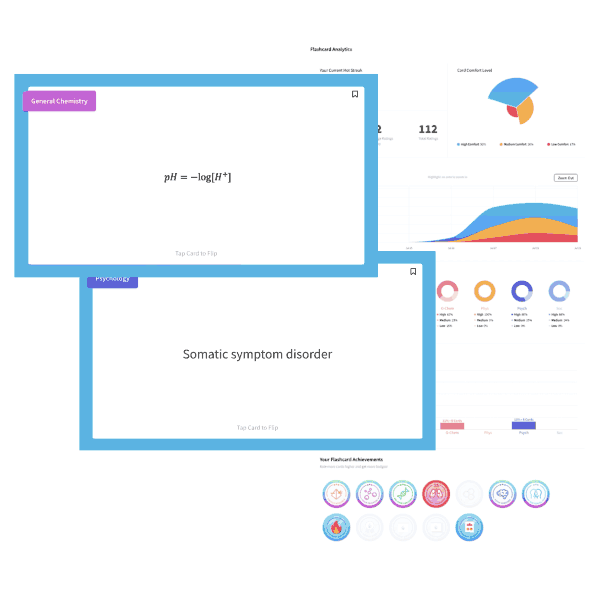
The ideal gas equation is written as PV=nRT, and the variables represent pressure, volume, number of moles, the ideal gas constant, and temperature (in Kelvin). One useful mnemonic is to sound out the law as “piv-nert.”
Changing one variable in the equation will bring about a compensatory change in another variable, all others held constant. Here, pressure and volume are held constant; the temperature must decrease to account for the increased number of moles “n” of gas.
A. increasing the number of moles of gas has no effect, incorrect, The ideal gas law must be obeyed leading to a decrease in temperature.
B. the temperature in the container increases, incorrect, This is the opposite effect from either incorrect algebra or starting formula.
C. the temperature in the container decreases, correct.
D. the effect of increasing the number of moles of ideal gas cannot be determined, incorrect, The gas in the container will follow the idea gas law.
Want more MCAT practice?
We’ve got options for every schedule and learning style!
From the best online MCAT course created by top instructors with 524+ MCAT scores to the most representative full-length practice exams and private tutoring, we can custom tailor your MCAT prep to your goals!
Not sure which option is right for you? Schedule a free MCAT consultation with an MCAT expert using the form below. No obligation, just expert advice.