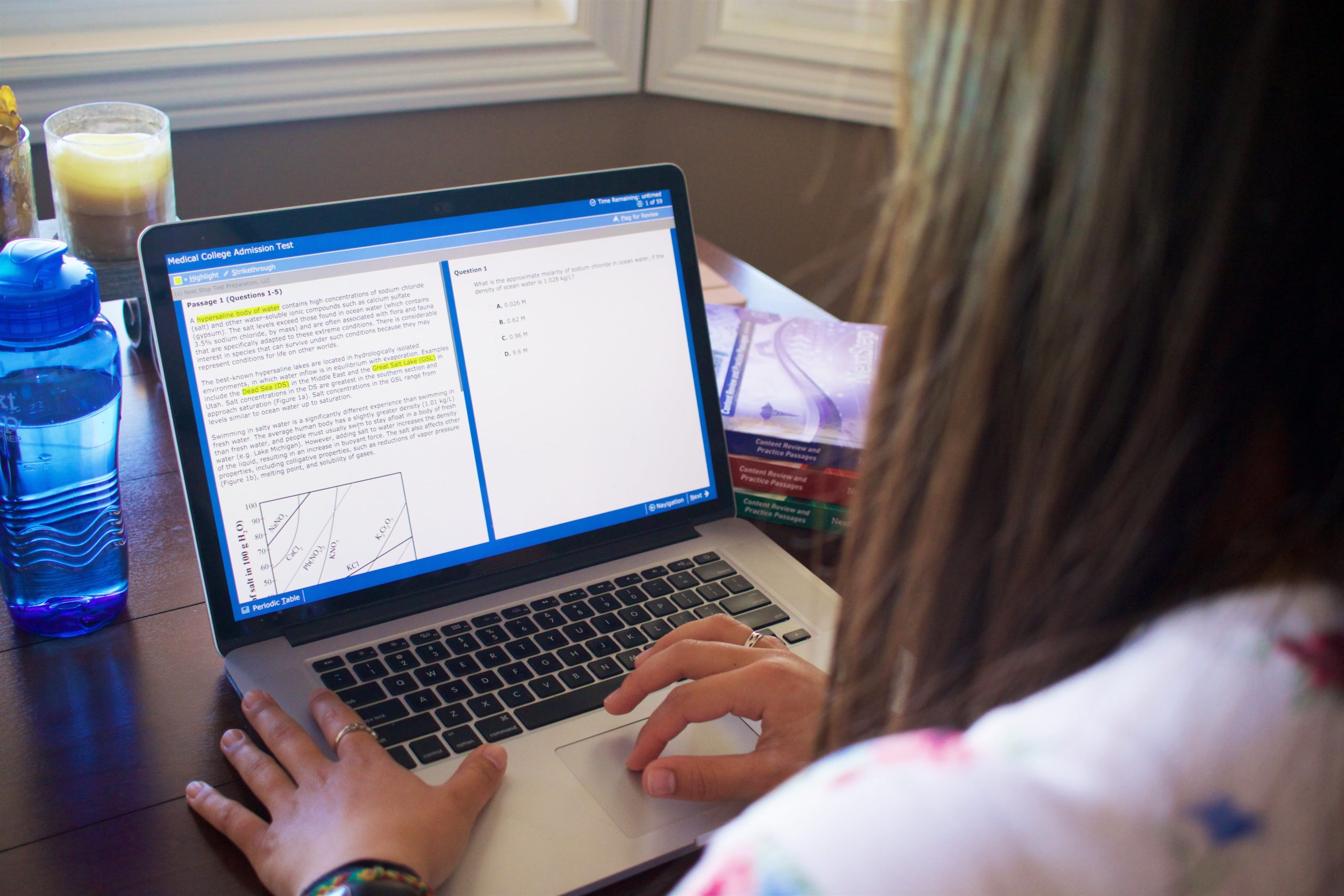
What is the most common electron configuration for Chromium (Cr)?
A. [Ne] 4s23d4
B. [Ar] 4s23d4
C. [Ar] 4s13d5
D. [Ne] 3d6
Click for Explanation
[Ne] indicates filled 2s and 2p orbitals, which is incorrect for chromium. [Ar] correctly indicates filled 3s and 3p orbitals. Normally, the 4s would fill with 2 electrons next, followed by 4 electrons in the 3d orbital. However, chromium is an exception which fills 4s1 and 3d5. Copper is a similar exception which fills 4s1 and 3d10 rather than 4s2 and 3d9. These are the two main exceptions you should know for the MCAT. Note that elements in the same periods as Cr and Cu (such as Au, Ag, and Mo) similarly serve as exceptions to the usual rule.
A. [Ne] 4s23d4, incorrect, The correct orbital shorthand is [Ar].
B. [Ar] 4s23d4, incorrect, While this would normally be true, Cr and Cu are exceptions which fill their 3d orbitals to 5 and 10 before placing their last electron in 4s1.
C. [Ar] 4s13d5, correct.
D. [Ne] 3d6, incorrect, [Ar] is the correct shorthand rather than [Ne], and the correct orbital filling is shown in answer c). Note that chromium is an exception to the orbital filling guidelines.
Want more MCAT practice?
We’ve got options for every schedule and learning style!
From the best online MCAT course created by top instructors with 524+ MCAT scores to the most representative full-length practice exams and private tutoring, we can custom tailor your MCAT prep to your goals!
Not sure which option is right for you? Schedule a free MCAT consultation with an MCAT expert using the form below. No obligation, just expert advice.
Search the Blog

Free Consultation
Interested in our Online MCAT Course, One-on-One MCAT Tutoring or Med admissions packages? Set up a free consultation with one of our experienced Senior Student Advisors.
Schedule NowPopular Posts
-
MCAT Blog What's on the MCAT?
-
MCAT Blog How to Review MCAT Full Lengths

Free MCAT Practice Account
Need great MCAT practice?Get the most representative MCAT practice possible when you sign up for our free MCAT Account, which includes a half-length diagnostic exam and one of our full-length MCAT practice exams.
Learn More