I can recall how dreadful it was to enter the MCAT testing room with simply an identification card and noise-canceling headphones, questioning whether you have the right tools to tackle the exam. Luckily, there are various resources provided during the MCAT exam that can guide you toward the right answers, including the periodic table for the MCAT.
Even though the MCAT rarely includes questions asking directly about periodic properties, knowing the periodic trends is fundamental to answering many multiple-choice questions in the science sections.
In this blog, we’re focusing on explaining periodic trends and discussing variations in the atomic radius, ionic radius, ionization energy, electron affinity, and electronegativity.
If you need a more in-depth look at the periodic trends for the MCAT, Blueprint Prep’s Self-Paced MCAT Course and Live Course students can attend Office Hour sessions specifically about this topic.
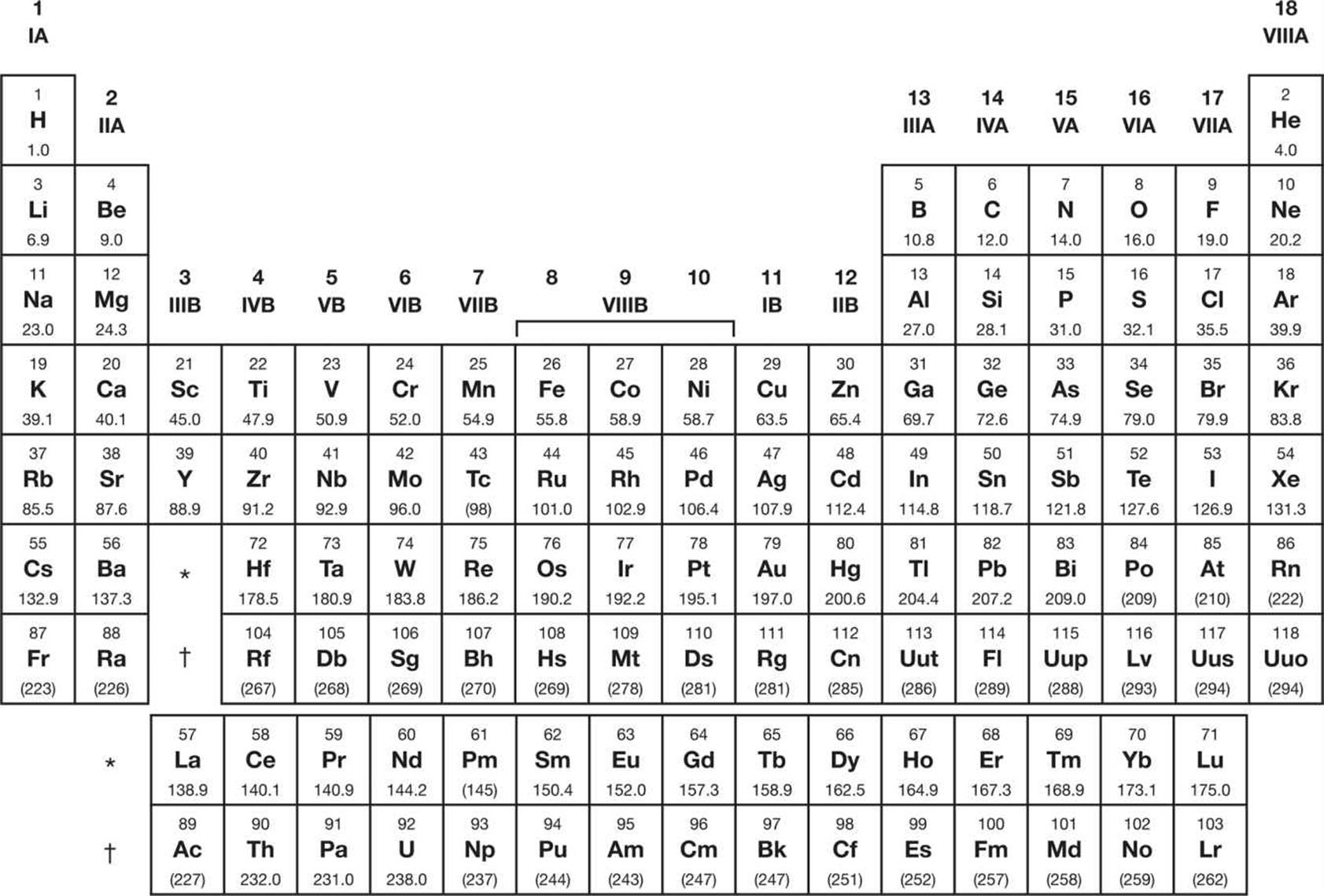
Understanding the Periodic Table for the MCAT

Image courtesy of schoolbag.info
Atomic and Ionic Radii
It’s not possible to specify a definite value for the radius of an isolated atom. Think of the atomic radius as half the single-bond length of a homonuclear bond. Atomic radii variations are closely related to the concept of effective nuclear charge.
Recall that the effective nuclear charge is the net positive charge experienced by valence electrons or the difference between the number of protons and the shielding electrons located in the energy levels between the nucleus and the valence electrons.
Visualize these shielding electrons as the ones that guard and “shield” the valence electrons from the attractive force of the positive nucleus. Strong “shielding” will not allow the valence electrons to be strongly pulled by the nucleus and they will be free to spread out, thus making the atomic radius bigger. Conversely, the greater the effective nuclear charge, the smaller the atomic radius.
When studying the periodic table for the MCAT, remember that shifting from left to right across the same period will increase the number of protons in the nucleus while keeping the number of shielding electrons constant since only the number of valence electrons increases across the same period. The effective nuclear charge is greater towards the right side and that is the reason why the atomic radius decreases as we shift from left to right.
The variation in atomic size of the elements in the same group can also be explained by the concept of electron shielding since going down a column increases the principal quantum number n and the number of orbitals between the nucleus and the valence electrons. The added inner shells are effective at shielding valence electrons which allows for distinct increases in size. Therefore, the atomic radius increases from top to bottom down a group.
The concept of effective nuclear charge and comparisons of ionic radii with atomic radii of the same element show that cations are always smaller than the parent atom since they are formed by losing electrons and anions are always larger than the parent atom since they are formed by gaining electrons.
Sign up to get expert tips and exclusive invites to free MCAT classes and medical school admissions workshops!
Ionization Energy
The periodic table also helps us predict the ionization energy which is the energy required to remove electrons from the highest-energy orbital of neutral atoms.
To remove electrons, work must be done to overcome the electrostatic attraction between the nucleus and the electrons. The ionization energy increases as the nuclear charge increases from left to right across the periodic table.
On the other hand, the ionization energy decreases while going down a column because electrons are removed from shells that are further from the nucleus and its attractive force.
This trend can also be explained in terms of electrostatic forces and Coulomb`s Law. The electrostatic force of the nucleus is inversely proportional to the radius squared.
As the radius increases, the electrostatic force decreases. Thus, the ionization energy decreases as well. It is important to know that an atom has as many ionization energies as it has electrons.
The second or third ionization energy is always bigger than the second or first, respectively. In addition, noble gases have the highest ionization energies due to their completed outer shells.
Electron Affinity
While ionization energy refers to the formation of positive ions, electron affinity refers to the formation of negative ions by adding electrons to the lowest vacant energy orbitals.
Since the added electron will be attracted by the nucleus, this is generally an exothermic process that releases energy. The electron affinity trends are not as clear as the ones for ionization energy or atomic radius. Electron affinities become more exothermic as we shift from left to right across a period and less exothermic as we go down a group from top to bottom.
Electronegativity
An electronegative atom is like a child who strongly pulls on their toys and is not willing to share them.
Electronegativity measures the tendency of atoms to pull and form bonds with electrons. It is helpful to think of electronegativity trends relative to the atomic radius and the attractive force of the nucleus.
An atom with a smaller atomic radius exerts a great electrostatic force on its valence electrons and is not willing to share them with other atoms in a covalent bond. Therefore, it has a higher electronegativity than atoms with larger radiuses that cannot exert the same attractive force on their outer electrons.
As we shift from left to right across the periodic table, the radius decreases and the atoms have a higher tendency to pull electrons into their valence shell. Conversely, as the radius increases from top to bottom down a column, it becomes harder for the nucleus to keep hold of electrons.
Electronegativity increases from left to right and decreases from top to bottom, except for noble gases, lanthanides, and actinides which do not possess electronegativity values. It is important to note that Fluorine, which sits on the top right corner of the periodic table, is the most electronegative element.
Final Thoughts
The MCAT periodic table and periodic trends might seem overwhelming, but with a little review of MCAT practice questions—either from a Qbank or practice exam—you’ll be able to understand their complexities.
If you need additional help, the Blueprint MCAT Self-Paced MCAT and Live Online Courses cover periodic elements in greater detail. For more one-on-one support, an MCAT tutor might be perfect for your MCAT strategy. Unable to decide? Schedule a free consultation with an MCAT Advisor to sort your through options and find the MCAT prep style that’s right for you.
Can’t wait to get started? Create a free Blueprint MCAT account to access our customizable study planner, free practice test with in-depth analytics, and more!
Written By: Kristiana Nasto, Blueprint MCAT Tutor
Search the Blog

Free Consultation
Interested in our Online MCAT Course, One-on-One MCAT Tutoring or Med admissions packages? Set up a free consultation with one of our experienced Senior Student Advisors.
Schedule NowPopular Posts
-
MCAT Blog What's on the MCAT?
-
MCAT Blog How to Review MCAT Full Lengths

Free MCAT Practice Account
Need great MCAT practice?Get the most representative MCAT practice possible when you sign up for our free MCAT Account, which includes a half-length diagnostic exam and one of our full-length MCAT practice exams.
Learn More