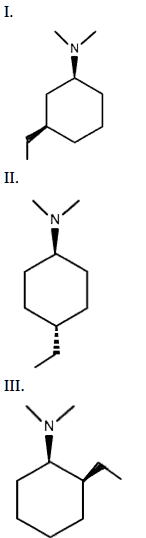
In the cyclohexane rings shown below, the tertiary amine is oriented in the equatorial position. Which of the following structures also has the ethyl group situated in the equatorial configuration?
- I only
- II only
- I and II
- I, II, and III
Explanation
This question tests your understanding of cyclohexane ring structures. The most stable conformation for a cyclohexane ring is the chair conformation with the bulkier substituent oriented in the equatorial configuration. As indicated in the question stem, the secondary amine is situated in this position, as expected.
In the compounds shown above, the carbon attached to the secondary amine will be designated as carbon one. The position of the ethyl group, indicated by cis or trans, will determine axial or equatorial attachment.
Compounds with the ethyl group attached to C3 or C5 in the cis configuration have the ethyl group in the equatorial position. Conversely, compounds with the ethyl group attached to C3 or C5 in the trans configuration have the ethyl group in the axial position. Additionally, compounds with the ethyl group attached to C2, C4, or C6 in the trans configuration have ethyl attached in the equatorial position. Lastly, compounds with the ethyl group attached to C2, C4, or C6 in the cis configuration have the ethyl group attached in the axial position.
This can be summarized as follows:
- C1 up = equatorial
- C2 up = axial
- C3 up = equatorial
- C4 up = axial
- C5 up = equatorial
- C6 up = axial
Compound I has the ethyl group in the up position on C3, thus equatorial.
Compound II has the ethyl group on the C4 position but pointed down, thus equatorial.
Compound III has the ethyl group on the C2 carbon point up, thus axial.
Therefore, C is the correct answer.
Next Step Test Preparation provides one-on-one MCAT tutor programs nationwide