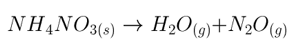Click for Explanation
This solution has 3 steps: balance the equation, determine the required number of moles of product and reactant, and calculate the mass of reactant necessary. The reaction as written is unbalanced. In the balanced equation, 2 moles of H2O and 1 mole of N2O are made for each 1 mole of NH4NO3. 3 moles of product gas result from each mole of reactant. 22.4 L of gas corresponds to 1 mole of gas at STP, and the specified 134.4 L indicates 6 moles of product gases. 6 moles of mixed product gas requires 2 moles of reactant. NH4NO3 weighs 80 g/mole, so 160 g are required.
A. 160, correct. 2 moles of NH4NO3 yields 4 moles of H2O and 2 moles of N2O, for 6 moles of mixed product gas occupying 134.4 L at STP.
B. 240, incorrect, This answer results from using the unbalanced equation as written.
C. 960, incorrect, This answer results from using the unbalanced equation and calculating the mass of 12 moles NH4NO3 for 6 moles N2O product rather than mixed gas.
D. 1440, incorrect, This answer results from using the correct balanced equation and calculating the mass of 18 moles of NH4NO3 for 6 moles of N2O product rather than mixed gas.
Want more MCAT practice?
We’ve got options for every schedule and learning style!
From the best online MCAT course created by top instructors with 524+ MCAT scores to the most representative full-length practice exams and private tutoring, we can custom tailor your MCAT prep to your goals!
Not sure which option is right for you? Schedule a free MCAT consultation with an MCAT expert using the form below. No obligation, just expert advice.
Search the Blog

Free Consultation
Interested in our Online MCAT Course, One-on-One MCAT Tutoring or Med admissions packages? Set up a free consultation with one of our experienced Senior Student Advisors.
Schedule NowPopular Posts
-
MCAT Blog What's on the MCAT?
-
MCAT Blog How to Review MCAT Full Lengths

Free MCAT Practice Account
Need great MCAT practice?Get the most representative MCAT practice possible when you sign up for our free MCAT Account, which includes a half-length diagnostic exam and one of our full-length MCAT practice exams.
Learn More