What is chromatography, and why does the MCAT care, you ask? Chromatography encompasses a wide range of methods used to separate and/or analyze complex mixes of chemical and biochemical molecules. The components to be separated are distributed between two phases: a stationary phase bed and a mobile phase which moves through the stationary phase.
While the MCAT will not expect us to be chromatography experts, the test makers expect us to know the fundamentals, how/when to apply appropriate techniques, and will expect us to make conclusions based on reported results. This is why we cover them extensively in our online MCAT course and with private MCAT tutors.
For the modern MCAT, test-relevant chromatography is centered on three properties:
- Charge
- Binding interactions
- Size
Ion Exchange Chromatography
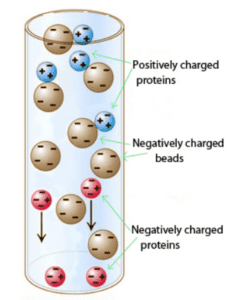
This technique is most often used to separate charged biomolecules such as amino acids, proteins, or nucleotides based on charge. Depending on the pH of their environment, proteins may carry a net positive charge, a net negative charge, or no charge. The pH at which a protein or amino acid has no net charge is called its isoelectric point (pI). The pI can be calculated based on the primary sequence of the molecule. The choice of buffer pH then determines the net charge of the target protein.
In a buffer with a pH > pI of the target protein, the protein will carry a net negative charge; so a positively charged anion exchange resin is chosen to capture this protein.
In a buffer with a pH < pI of the target protein, the protein will carry a positive net charge; so a negatively-charged cation exchange resin is chosen.
When an ion exchange chromatography column is loaded with a sample at a particular pH, all proteins that are appropriately charged will bind to the resin. For example, if a cation exchange resin is chosen, all proteins that are positively charged will bind to the negatively charged column beads. This proper matching of bead charge to target molecule charge is a favorite of the MCAT, since it requires us to recognize the charge of molecules based on the identity of amino acid composition.
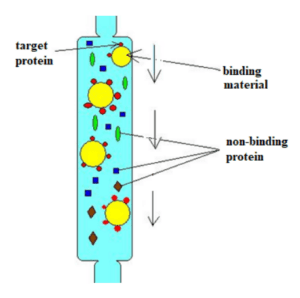
Affinity Chromatography
This method separates biomolecules based on a specific binding interaction between a ligand and a binding partner. MCAT examples of applying this technique include enzyme and substrate, antibody and antigen, and enzyme and inhibitor pairings. An important part of affinity chromatography design is choosing the proper binding material, so be on the lookout for any clues the tests gives us as to the interactions used by the target molecules.
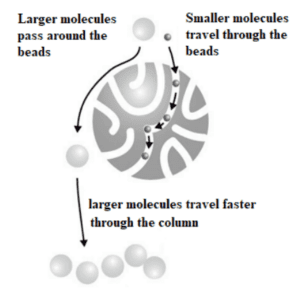
Size Exclusion Chromatography
This method separates biomolecules based on their size. A mixture (including the target molecules) is filtered through a gel, inside of which are spherical beads containing pores of a specific size. Molecules of different sizes are included or excluded from the pores within the gel. Smaller molecules diffuse into the pores and their flow through the column is retarded according to their size, while larger molecules do not enter the pores and are eluted in the column’s void volume. As they pass through the column, molecules are eluted in order of decreasing molecular weight.
Even very similar components, such as proteins that may only vary by a single amino acid, can be separated with chromatography. These techniques can be used to purify any appropriate substance if the right adsorbent material, carrier fluid, and conditions are used. In medicine, chromatography is advantageous since it can separate even fragile molecules. As a result, chromatography is suited to a variety of uses in multiple medically-relevant scenarios the exam loves to test. Always be aware of what properties we are given about the molecules being discussed, and we will be able to earn points on questions about biotechnology on test day.