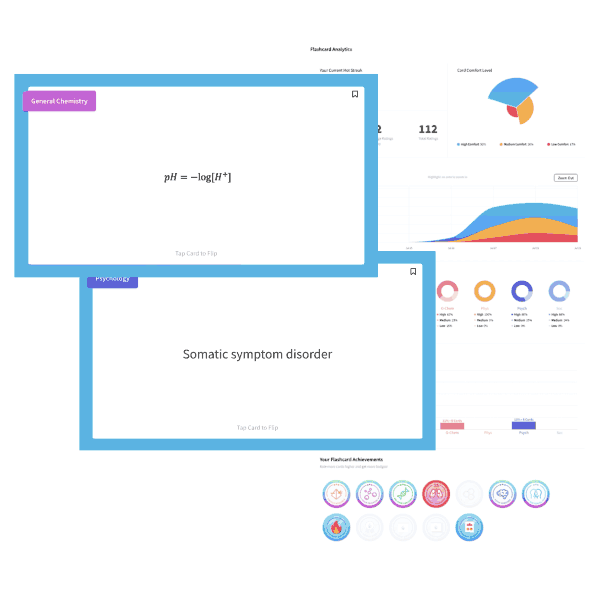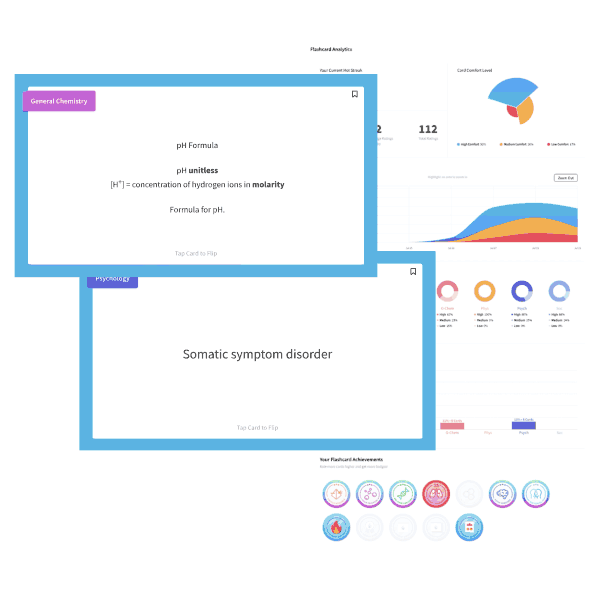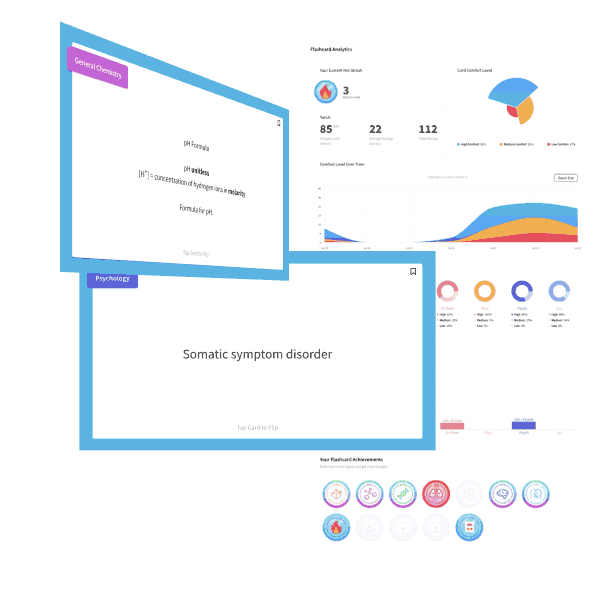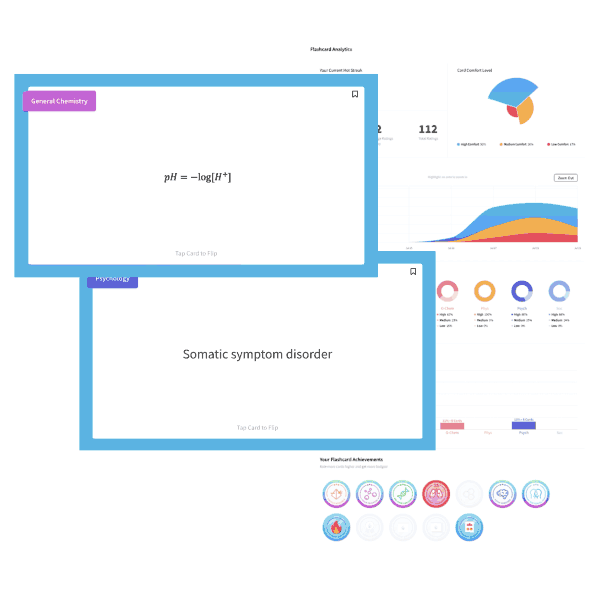
A NaOH solution has an OH– concentration of 1×10-5M. What is the pH of the solution?
A. 2
B. 5
C. 9
D. 12
Click for Explanation
Two relationships are necessary for this solution:
pOH = -log[OH–] and pOH + pH = 14
pOH = -log[1×10-5] = 5, and 14 – 5 = 9.
A. 2, incorrect, This results from subtracting the pOH from 7.
B. 5, incorrect, This is the pOH.
C. 9, correct.
D. 12, incorrect, This results from adding the pOH to 7.
Want more MCAT practice?
We’ve got options for every schedule and learning style!
From the best online MCAT course created by top instructors with 524+ MCAT scores to the most representative full-length practice exams and private tutoring, we can custom tailor your MCAT prep to your goals!
Not sure which option is right for you? Schedule a free MCAT consultation with an MCAT expert using the form below. No obligation, just expert advice.