Which of the following characterizes butane?
- high solubility in water
- strong dipole moment
- high molecular weight
- low solubility in water
Click for Explanation
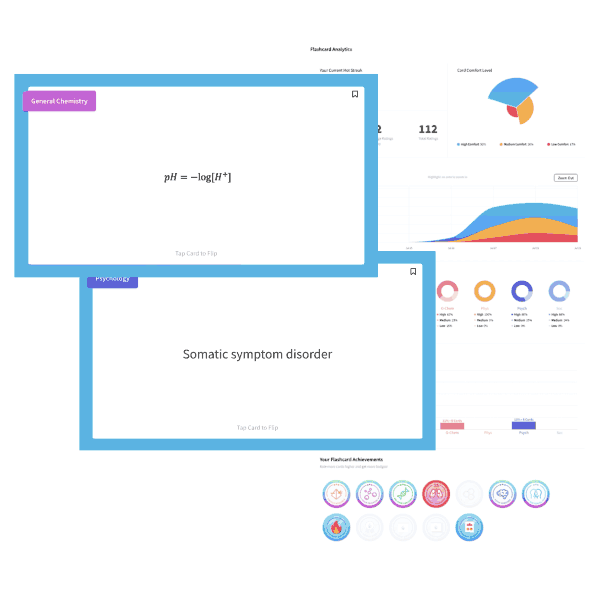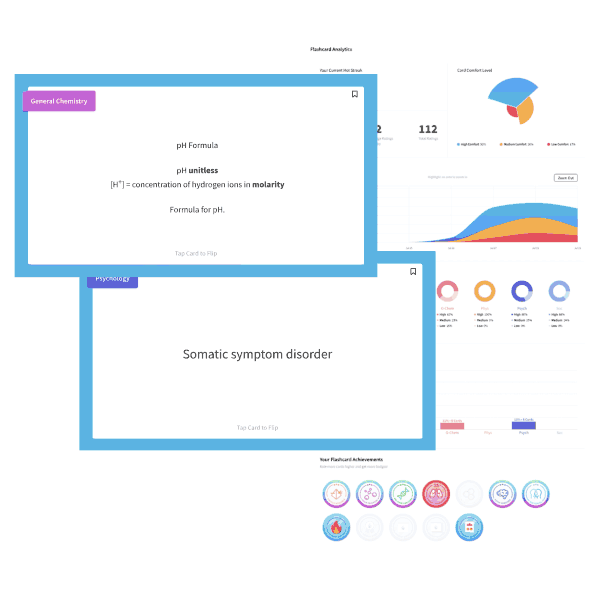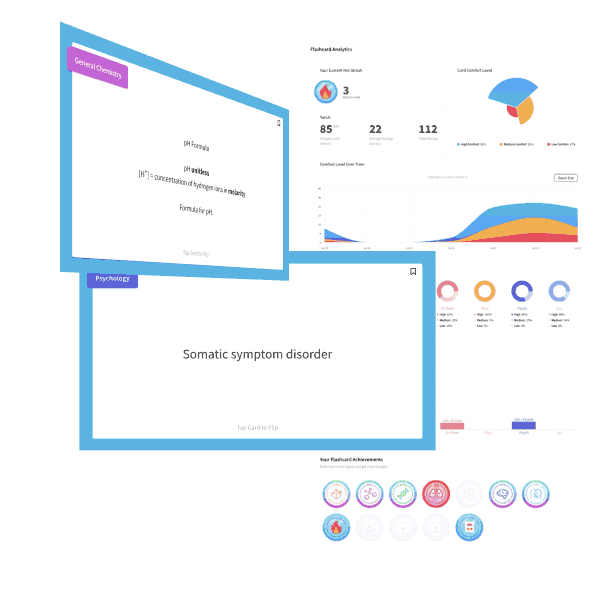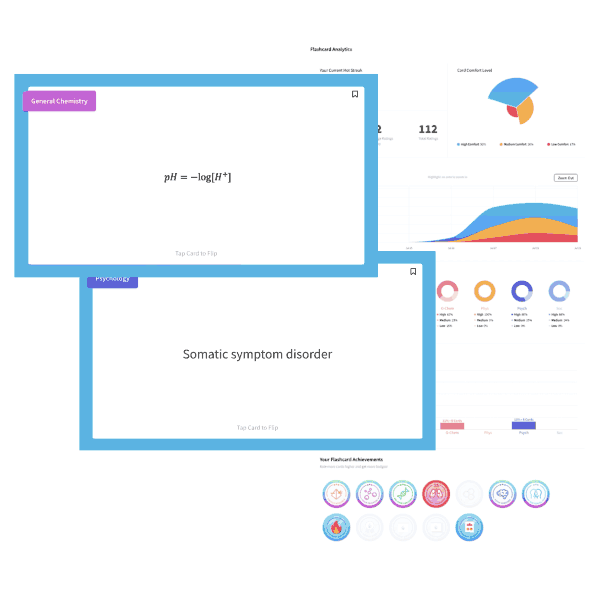
Butane is a four carbon alkane. As such, it is non-polar and low in molecular weight. Solubility in water is primarily governed by polarity and ability to hydrogen-bond, both of which butane lacks. Choice d is the best choice, low solubility in water.
A .high solubility in water, incorrect, Nonpolar compounds do not dissolve well in water.
B. strong dipole moment, incorrect, Alkanes lack dipole moments.
C. high molecular weight, incorrect, A four carbon alkane has low molecular weight. High molecular weight on the MCAT is often used to describe long-chain polymers and very large molecules, and butane does not qualify for either designation.
D. low solubility in water, correct.
Want more MCAT practice?
We’ve got options for every schedule and learning style!
From the best online MCAT course created by top instructors with 524+ MCAT scores to the most representative full-length practice exams and private tutoring, we can custom tailor your MCAT prep to your goals!
Not sure which option is right for you? Schedule a free MCAT consultation with an MCAT expert using the form below. No obligation, just expert advice.
MCAT is a registered trademark of the Association of American Medical Colleges (AAMC), which is not affiliated with Blueprint.
