Blueprint MCAT Blog: MCAT Chemistry
Categories
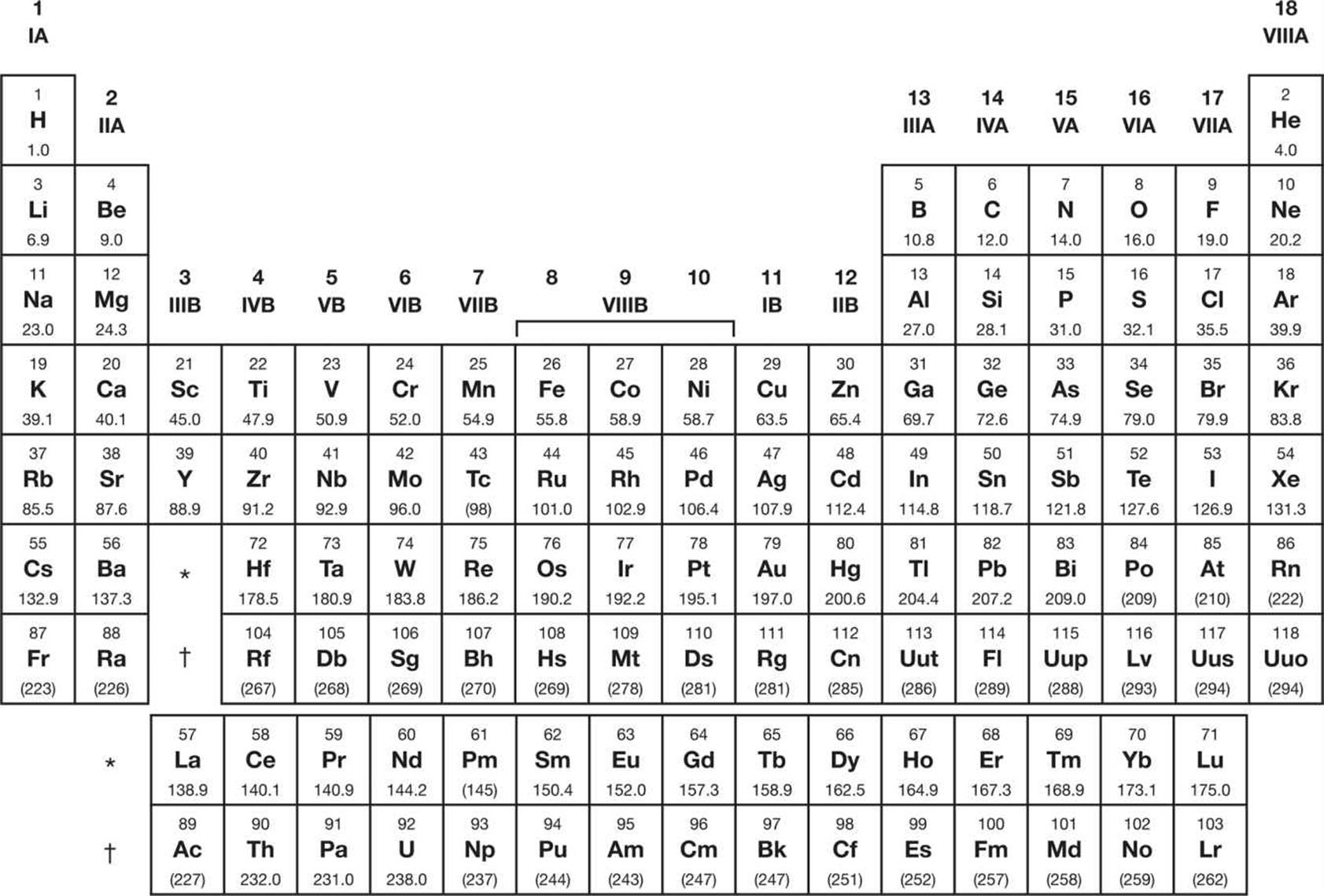
Oct 22, 2019
How to Understand the MCAT Periodic Table & Trends
Learn how to predict element properties and efficiently use the periodic table for the MCAT. READ MORE
May 22, 2019
Identifying Methodological Flaws in Studies
One of the things that really surprises students about the MCAT is the heavy focus on how experiments are set up and ran. This can really catch a student off-guard, as most undergrad courses don’t really emphasize this. But from the point of view of the AAMC, it’s obvious that they want to test this. In order to be a good doctor, you need to be able to understand the quickly changing research that is coming from our more... READ MORE
Jan 16, 2018
Organic Chemistry on the MCAT – Focus on the Fundamentals
Organic chemistry is one of the most common subjects that students identify as a source of anxiety when preparing for the MCAT. However, on average, it will only comprise ~5% of the test: 15% of the Chemical and Physical Foundations section, 5% of the Biological and Biochemical Foundations Section, and 0% of the remaining two READ MORE
Jul 01, 2017
MCAT Chemistry – Solubility Constants and the Common Ion Effect
Of the general chemistry content covered on the MCAT, solubility is notable because it seems simple, but contains some surprises. On one hand, solubility is an accessible topic to anyone who has ever added sugar to their coffee or tea, but on the other hand, there are some formal aspects of how it’s analyzed that READ MORE
Jun 01, 2017
Types of Inhibition: Focus on the Basics
Michaelis-Menten enzyme kinetics, and especially keeping track of the various kinds of reversible enzyme inhibition, is one of the most challenging areas of biochemistry for many MCAT students. It’s extremely easy to get lost in the details of memorizing terminology, types of graphs, x-intercepts, y-intercepts, and so forth – but superficially memorized information is very READ MORE
Jan 20, 2017
Le Châtelier and You: The Principle That Ties Together All Things Chemistry
When preparing for the MCAT, it’s important to identify topics that cut across content areas, because conceptual intersections between fields are (1) relatively likely to be high-yield topics and (2) help you integrate the material in a way that will allow you to apply the content flexibly in new contexts, as is often necessary for READ MORESearch the Blog

Free Consultation
Interested in our Online MCAT Course, One-on-One MCAT Tutoring or Med admissions packages? Set up a free consultation with one of our experienced Senior Student Advisors.
Schedule NowPopular Posts
-
MCAT Blog What's on the MCAT?
-
MCAT Blog How to Review MCAT Full Lengths

Free MCAT Practice Account
Need great MCAT practice?Get the most representative MCAT practice possible when you sign up for our free MCAT Account, which includes a half-length diagnostic exam and one of our full-length MCAT practice exams.
Learn More




