As is with most topics on the MCAT, you won’t be surprised to learn that we interact with electrochemical cells every day: when we use our cellphones, change the channel on the TV, and even when the nerve cells in our body are firing. When you can separate a redox reaction into two separate half-reactions, you can create an electrochemical cell that generates electrical energy.
This guide goes over three of the most important things to understand about electrochemical cells for success on the MCAT.
3 Concepts To Know About Electrochemical Cells for the MCAT
1. Half-Reactions
In a redox reaction, one species is oxidized and another species is reduced (as a reminder, oxidation is the loss of electrons and reduction is the gain of electrons (OIL RIG and LeO goes GeR are great MCAT mnemonics for this!). These redox reactions can be written as half-reactions.
Consider the following redox reaction:
Zn(s)+Cu2+→Zn2++Cu(s)
This can be written as two half-reactions in which zinc is oxidized and copper is reduced. The common notation is to write the oxidation half-reaction on the left and the reduction half-reaction on the right:
Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
Zinc and copper solids are considered conductive electrodes (zinc is the anode and copper is the cathode).
2. Galvanic Cells
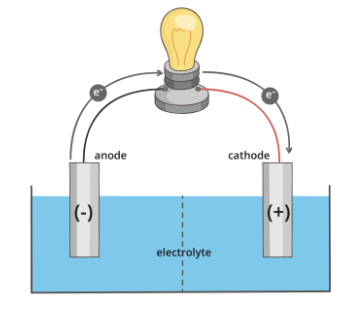
Galvanic cells are spontaneous electrochemical cells. A standard galvanic cell contains two metal electrodes immersed in a solution of the dissolved salt of the corresponding metal. Each solution represents one redox half-reaction, and the two solutions are connected by a salt bridge which prevents charge buildup as shown below:
©2005-2023 Blueprint Test Preparation LLC. All Rights Reserved.
Now let’s consider a galvanic cell measuring the cell potential of the following redox reaction:
Zn(s)+Cu2+→Zn2++Cu(s).
The anode would be the zinc electrode, and the cathode would be the copper electrode. As zinc is oxidized, the excess electrons will travel through the wire and act as an electrical current to power the lightbulb. The electrons will then reduce copper and complete the circuit. Ultimately, the copper electrode will increase in mass as Cu2+ ions are reduced to Cu(s) and deposited on the electrode.
3. Electrolytic Cells
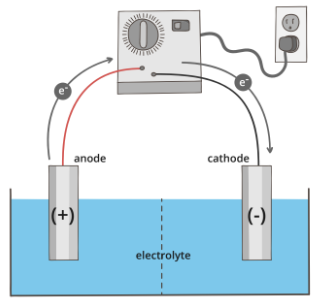
While galvanic cells are powered by spontaneous redox reactions, electrolytic cells require an input of energy to drive non-spontaneous redox reactions. You can think about this like a reverse voltage of a galvanic cell, like when you charge your phone battery back up after using it all day. A major difference between electrolytic and galvanic cells is that in electrolytic cells, the two electrodes can be submerged in one solution.
Additionally, the anode will become the positive electrode despite remaining as the site of oxidation, and the cathode will become the negative electrode, as shown below:
 ©2005-2023 Blueprint Test Preparation LLC. All Rights Reserved.
©2005-2023 Blueprint Test Preparation LLC. All Rights Reserved.
Need More Help With Electrochemistry Questions on the MCAT?
If you need more help with electrochemistry or any other MCAT topics, our experts are here to help! Whether you need the flexibility of a Self-Paced Course, the instruction of a live 515+ Course, or the 1:1 attention of a private MCAT tutor, Blueprint MCAT has the MCAT prep option that works for your learning style!
Start today with a free MCAT diagnostic, one free practice exam, and tons more MCAT prep resources.